The use of lithium batteries is restricted in low temperature environments. Except for the severe deterioration of discharge capacity, lithium batteries cannot be charged at low temperatures. During low-temperature charging, the intercalation of lithium ions on the graphite electrode of the battery and the lithium plating reaction coexist and compete with each other. Under low temperature conditions, the diffusion of lithium ions in graphite is inhibited, and the conductivity of the electrolyte decreases, resulting in a decrease in the intercalation rate and the lithium plating reaction on the graphite surface is more likely to occur. The main reasons for the decrease in the life of lithium-ion batteries when used at low temperatures are the increase in internal impedance and the degradation of the capacity due to the precipitation of lithium ions.
1. The influence of low temperature on battery discharge capacity
Capacity is one of the most important parameters of a lithium battery. The curve of its size with temperature is shown in the figure below. The figure below is the discharge curve of a lithium iron phosphate battery. For lithium iron phosphate batteries, the end-of-charge voltage is 3.65±0.05V, and the end-of-discharge voltage is 2±0.05V. The two curves are the temperature capacity curves obtained when the battery is discharged at different temperatures at 0.1C and 0.3C. Obviously, the capacity gradually increases as the temperature rises, and the capacity at -20°C is only about 60% of the capacity at 15°C. In addition to capacity, the open circuit voltage of the battery decreases with temperature. We all know that the energy contained in the battery is the product of the capacity and the terminal voltage. When both multipliers are reduced, the energy in the battery must be the superposition of the reduction effects of the two.
At low temperatures, the activity of the cathode material decreases, so that the number of lithium ions that can move and bring about the discharge current decreases, which is the root cause of the decrease in capacity.
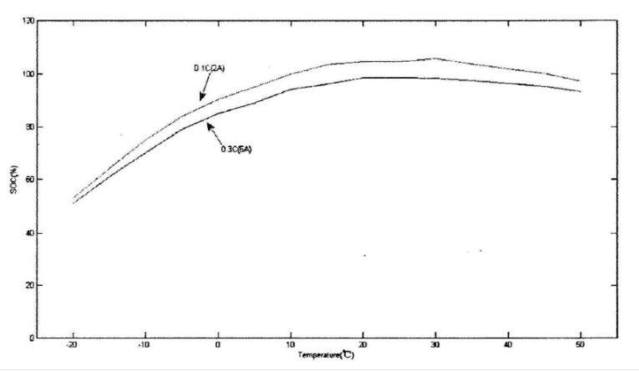
Lithium battery discharge capacity at different temperatures and discharge rates
2. The influence of low temperature on battery internal resistance
The relationship between lithium battery temperature and resistance is shown in the figure below. Different curves represent different charge levels of the battery itself. Under any amount of charge, the internal resistance of the battery increases significantly as the temperature decreases. The lower the charge, the greater the internal resistance, and this trend also remains unchanged as the temperature changes.
At low temperature, the diffusion and movement ability of charged ions in the positive and negative materials becomes poor, and it becomes difficult to pass through the passivation film of the electrode and the electrolyte, and the transmission speed in the electrolyte is also reduced, and additional production will be generated during the transmission process. A lot of calories. After lithium ions reach the negative electrode, the diffusion inside the negative electrode material also becomes unsmooth. Throughout the process, the movement of the charged ions has become difficult. From the outside, it is the internal resistance of the battery that has increased.
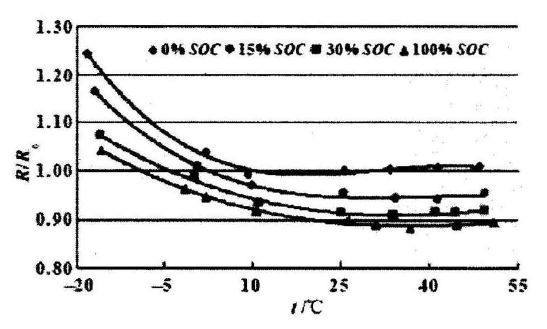
The relationship between internal resistance and SOC and temperature
3. The influence of low temperature on battery charge and discharge efficiency
The following curve is the curve of charging efficiency following temperature change. We can observe that the charging efficiency at -20°C is only 65% of that at 15°C. I only talk about efficiency here. The harm of low-temperature charging is very serious, so I won't discuss it here. The low temperature brings about the various electrochemical performance changes described above, and the internal resistance increases significantly. During the discharge process, a large amount of electric energy is consumed on the internal resistance to heat up. The Coulomb efficiency we observed has decreased. When an electric car is driving, you will feel that the battery life seems to be about the same, and the battery life becomes shorter at low temperatures.
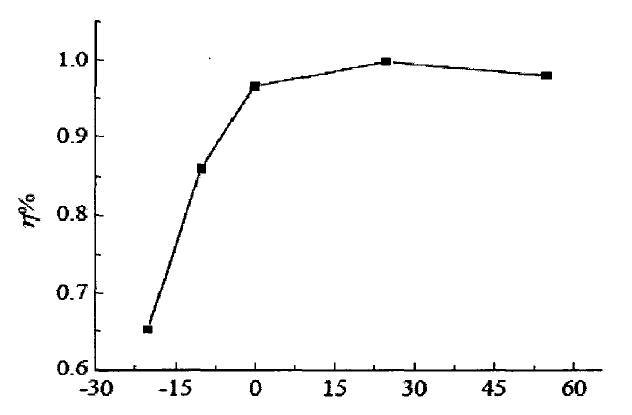
Trend chart of charging efficiency with temperature
4. Side reactions inside lithium-ion batteries
The performance of lithium batteries deteriorates severely at low temperatures, and some side reactions occur during the charging and discharging of lithium-ion batteries. These side reactions are mainly the irreversible reaction between lithium ions and the electrolyte, which will cause the capacity of the lithium battery to decline and further deteriorate the battery performance.
The consumption of conductive active materials causes capacity degradation. Taking into account the potential of the positive and negative electrodes in the battery, these side reactions are more likely to occur on the negative side than the positive electrode. Because the potential of the negative electrode material is much lower than that of the positive electrode material, the side-reaction deposits of ions and electrolyte solvent are deposited on the surface of the electrode, forming an SEI film. The impedance of the SEI film is one of the factors that cause the negative electrode to react over potential. When the battery is further cyclically aging, due to the continuous insertion and extraction of lithium ions on the negative electrode during continuous cycles, the expansion and contraction of the electrode will cause the SEI film to rupture. The cracks after the SEI membrane rupture provides a direct contact channel between the electrolyte and the electrode, thereby forming a new SEI membrane to fill the cracks and increase the thickness of the SEI membrane. These reaction processes continue to occur repeatedly as the battery is continuously charged and discharged, so that lithium ions are continuously reduced in the reaction, resulting in the decline of the discharge capacity of the lithium ion battery.
When charging, the deposit formed on the surface of the active material increases the resistance. The effective surface area of the active particles is reduced, and the ion resistance is increased. The available capacity and energy of lithium batteries are declining at the same time. Lithium batteries are more prone to side reactions during the charging process. When the lithium battery starts to charge, the lithium ions move to the negative electrode through the electrolyte, so the potential difference between the electrode and the electrolyte is reduced, making the lithium ions and the substances in the electrolyte more prone to irreversible side reactions. Different electrode materials of lithium-ion batteries have different curves of the relationship between its potential and the concentration of lithium intercalation of the electrode material.
5. Lithium battery low temperature preheating technology
Faced with the limited use of lithium batteries at low temperatures, the response strategy that technicians found is charging and preheating. Although it is a stopgap measure, it has obvious effects on improving the discharge capacity and long-term life of lithium batteries.
Before charging or using the lithium battery in a low temperature environment, the battery must be preheated. The way that the battery management system (BMS) in electric vehicles heats the battery can be roughly divided into two categories: external heating and internal heating. External heating methods include air heating, liquid heating, phase change material heating, and heat resistance heater or heat pump heating. These heating methods are generally located in the battery pack, or set in the container of the thermal cycle medium. The internal heating method heats the battery by exciting the electrochemical substances inside the battery through alternating current, so that the battery itself generates heat.
External heating
Regarding the method of heating with air, some researchers have conducted experiments using batteries and a set of atmospheric simulation systems. The experimental results show that batteries with heated surrounding air can release more capacity than batteries exposed in a low-temperature environment.
Compared with air heating, liquid heating has better thermal conductivity and higher heat conversion efficiency. But liquid heating requires a more complicated heating system. There have been many practical cases of the application of liquid heating in electric vehicles and hybrid vehicles. For example: in a Chevrolet Volt car, the heat exchange fluid surrounding the battery pack is heated by a 360V heater.
Phase change material heating batteries have also been used. When the battery temperature drops to the phase change temperature point of the phase change material, the heat stored in the phase change material will be released to keep the ambient temperature constant, that is, heat is transferred to the battery pack. The main advantage of phase change materials is that they can be used in environments with relatively rapid temperature changes.
Internal heating
Compared with external heating, AC excitation heating is another commonly used heating method, which is simpler in structure design, which is to heat the battery through alternating current. It does not need to design the heat transfer structure, but loads the positive and negative electrodes of the battery with a certain frequency of AC excitation, and the excitation acts on the electrochemical substances in the battery, which is equivalent to the effect of cyclically reciprocating small amplitude charge and discharge.
Compared with direct current heating current, alternating current or positive and negative square wave current can heat the battery during both the discharge and charge cycles, causing the battery temperature to rise, while the battery state of charge (SOC) is basically unchanged. Because of these characteristics, the AC internal preheating method has become a field of more research. In 2004, a foreign researcher first proposed to use alternating current to directly heat lithium-ion batteries, only using the internal resistance effect of the battery to generate heat. They did some tests on different batteries in different SOC states and at different temperatures (-20℃~40℃). The test results show that under a certain rate of current, all batteries will quickly generate heat.
A team in the United States conducted a study on the influence of heating frequency on heating effect. They conducted simulations at different frequencies from 0.01Hz to 2KHz, and compared the results with external heating methods. They believed that internal heating has obvious advantages.
Compared with the external heating method, the internal heating avoids long-path heat conduction and the formation of local hot spots near the heating device. Therefore, internal heating can heat the battery more uniformly with higher efficiency to achieve better heating effect and easier to achieve.
At present, most researches on internal AC preheating schemes focus on heating speed and efficiency, and heating strategies rarely have clear considerations for preventing side reactions such as lithium deposition. To prevent the generation of lithium deposition during the preheating process, BMS needs to be able to estimate and control the conditions of lithium deposition in real time. A model-based control battery heating technology at low temperatures is needed to achieve the above functions. With the development of new energy vehicles, the use of power lithium batteries is increasing day by day. The use of lithium batteries at low temperatures urgently needs to solve the battery preheating problem, which is a field very close to practical applications.



